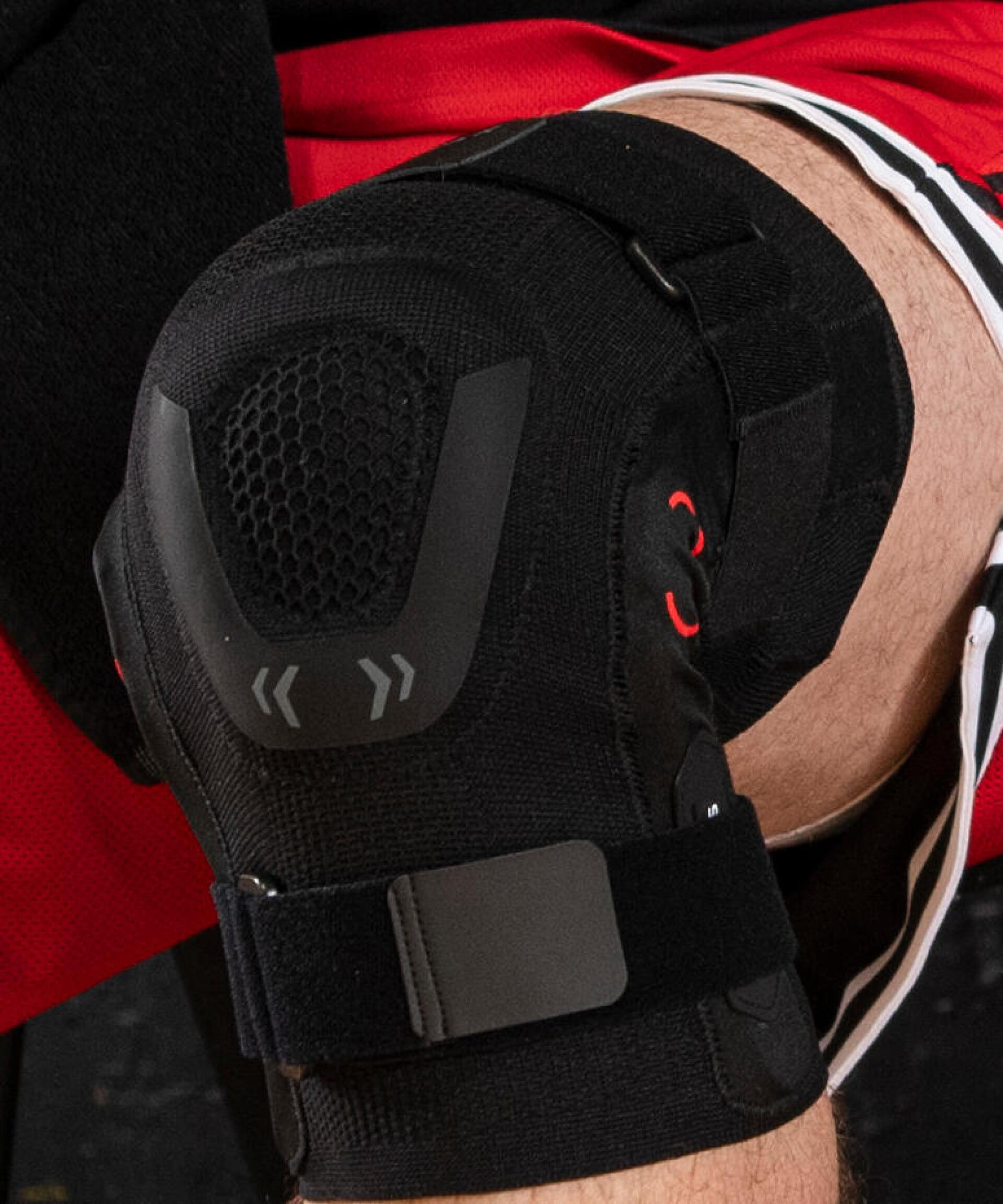
What is a knee protection certified as a medical device?
The supporting capability of our products is tested and verified mechanically. Testing protocols using integrated sensors measure the pressure exerted by the knee protections. The compression must reach certain minimum thresholds to be considered effective or optimum and to justify the claims made to customers.
For the Knee Strong 500 and 900 ranges designed for a return to sporting activity, our teams work in close cooperation with the French 'Ecole Nationale Supérieure des Arts et Métiers' which specialises in mechanical engineering. Engineers at this renowned higher-education establishment possess mechanical legs on which they simulate injuries, such as those affecting the cruciate ligaments, to establish the effectiveness of our products. This enables us to be confident in the functional capabilities of our knee protections.
At the same time, several research institutions, such as exist in the teaching hospitals in Lille and Boulogne-sur-Mer, carry out clinical trials to enable our products to be classified as medical devices.
Finally, our design teams work with three physiotherapy centres conducting similar clinical trials. Patients suffering from minor pains in the knee test the effectiveness of the knee protections, and benefit from a medical follow-up.